A new organic photosensitizer for the study of substitution and performance of dye-sensitized solar cells: oxindole bridged receptor
|
A new organic photosensitizer for the study of substitution and performance of dye-sensitized solar cells: oxindole bridged receptor |
|
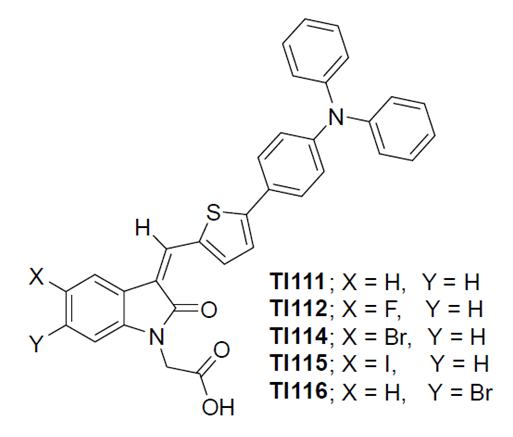
Prof. Chan-chin Su of Molecular Science and Engineering The use of photovoltaic technology directly from solar high-power resources makes the use of sunlight to collect energy become one of the important basic strategies for global energy production. The use of environmentally friendly and low-cost materials as solar energy generation devices has become a subject of interest in both academic and industrial research. The dye-sensitized solar cell (DSSC) proposed by O’Regan and Grätzel has thus attracted great attention. DSSC technology has shown great potential and can be used as an auxiliary tool for high efficiency, low production cost, and clean energy. To achieve excellent solar photovoltaic conversion efficiency in DSSC, considerable attention has been focused on the design and synthesis of various photosensitizers. In the past few decades, photosensitizers such as ruthenium (II) polypyridyl complexes and zinc-based porphyrin dyes have become DSSC effective photosensitizers due to their excellent photoelectric conversion efficiency. But the price, toxicity, and limited availability of metals, especially ruthenium, inspired researchers to seek alternative sensitizing dyes for DSSC. On the other hand, metal-free organic photosensitizers have attracted much attention due to their strong usability, flexibility in structural adjustment, and high molar extinction coefficient, which makes them competitive alternatives to other metal-based photosensitizers. Generally, metal-free organic photosensitizers are equipped with a donor-π-bridge-acceptor structure. According to reports in the literature, in the past, the modification of the donor and the π-bridge portion of the organic photosensitizer have been intensively studied, but there have been only a few advances in the acceptor group. The most commonly used acceptor (anchor group) is a carboxylic acid or cyanoacrylic acid. However, it has been reported that the cyanoacrylic acid acceptor has some disadvantages, such as reduced photostability due to the presence of double bonds, and has a major redox characteristic due to the strongly electronegative nitrile moiety. There are also reports that the nitrile group of cyanoacrylic acid will affect the acidity of the adjacent carboxylic acid, which will negatively affect the binding to the surface of TiO2. There have also been many attempts in the literature to replace strong electron-withdrawing cyano groups and construct new acceptor systems to achieve high absorption rates and good photostability. The acceptor portion of the dye shows a significant effect on the spectral response and device performance. Therefore, it is necessary to further explore various receptors and expand the "design principles" to help the discovery of metal-free photosensitizers. The surface laboratory of the molecular science and engineering department of Taipei Tech and together with the bioorganic laboratory of the chemistry department of the Central University have designed and synthesized a series of new oxyindole photosensitizers (TI111-TI116, left picture). The results showed the considerable photoelectric conversion efficiency for these new photosensitizers. These photosensitizers have excellent electron donor triphenylamine (TPA) donors and thiophene in the spacer and are distinguished by various halogen-substituted oxindole acceptors. The electronegative fluorine (F) element has been widely used in the field of organic light-emitting diodes and the synthesis of artificial amino acids. The change in the Fermi level of the photosensitizer can be achieved by using F substituents in its receptor, and the open-circuit voltage of the photosensitizer can be increased by reducing the dark current. We doped F atoms into the parent compound TI111 to form TI112 to change the photophysical properties of oxindole dyes. A comparative study of different halogen substituents in the donor portion of the photosensitizer is also evaluated. We have further expanded the scope of our research and designed TI114 and TI115, which incorporate bromine (Br) and iodine (I) substituents, respectively. We also found that the change of the position of the substituent relative to the anchor group can provide different battery performance (TI114 analog TI116).
|
 |
 |
|
Chemical structure of a new oxyindole photosensitizer(TI111-TI116, left) |
Member of the Surface Laboratory of the Molecular Department of our school and the Bioorganic Laboratory of the Chemistry Department of Central University |